

Image source: Apple Inc.
Apple Watch Series 4 will feature two new medical apps marketed with FDA’s blessing, thanks to De Novo classifications. The new watch will offer an ECG app, a software-only mobiles medical application that can classify whether there are signs of atrial fibrillation (AFib), and another software-only mobiles medical app analyzing pulse rates for irregular rhythms. (Neither is intended to replace "traditional methods of diagnosis or treatment," FDA stated in the notices.) These features are scheduled to be available in the United States later this year, Apple COO Jeff Williams said during the company’s September 12 launch event.
And, with a next-generation accelerometer and gyroscope, Series 4 can now detect falls to alert consumers and summon emergency services if needed.
These features (along with enhanced design, aesthetics, and other consumer conveniences) can allow consumers to create what Williams called “the ultimate health and fitness watch.”
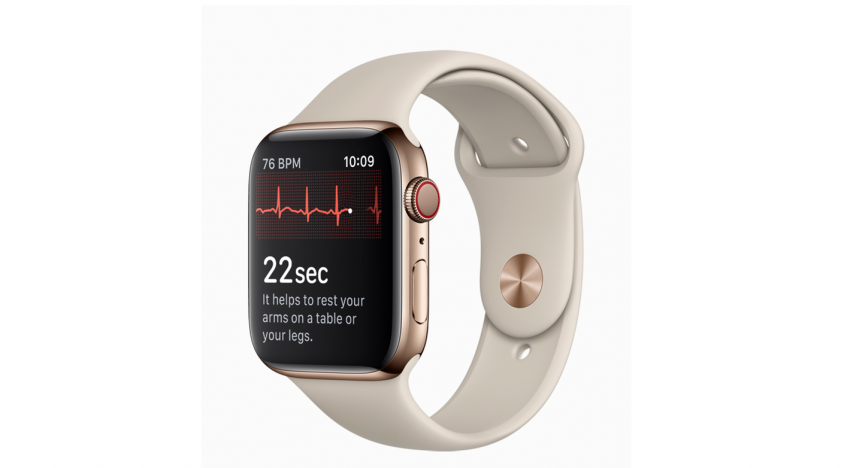
Apple Watch has offered an “optical heart sensor” from its beginnings, he said, but “we wanted to do even more in this space, so we are launching three new heart features.” Powered by that optical heart sensor, Apple Watch Series 4 will be able to detect and notify users of a low heart rate and of an irregular heart rhythm. A new electrical heart sensor uses new electrodes added to the watch’s back crystal and digital crown to allow consumers to take an electrocardiogram (ECG).
“The entire process takes just 30 seconds, and at the completion of the ECG recording, you will receive a heart rhythm classification,” Williams said. All ECG recordings, their classifications, noted symptoms are stored and can be shared with a doctor, he added, calling the built-in electrical heart sensor an “industry first.”
For detecting falls, the next-gen accelerometer and gyroscope feature “twice the dynamic range, measuring up to 32 g-forces, and can sample motion data 8 times faster,” he said. The technology allows Apple “to do something new. One of the leading causes of injuries worldwide is falls . . . . now Apple Watch Series 4 can detect a fall,” he said. Apple conducted studies with thousands of consumers to better understand motion patterns and more to develop the fall-detection capabilities.
Apple Watch Series 4 “is the ultimate guardian for your health, the best fitness companion, and the most convenient way to stay connected,” Williams said.
FDA weighed in on the advances the same day, issuing a joint statement on “agency efforts to work with tech industry to spur innovation in digital health” from FDA Commissioner Scott Gottlieb and CDRH Director Jeff Shuren. “Owing to digital advances, we’re experiencing a reimagination of health care delivery. Consumers are now empowered to take more control of their own health information to make better informed decisions about their medical care and healthy living. These advances enable better health outcomes for patients,” they wrote. “With these advances has come a new swath of companies that are investing in these new opportunities. These firms may be new to health care products and may not be accustomed to navigating the regulatory landscape that has traditionally surrounded these areas. A great example is the announcement of two mobiles medical apps designed by Apple to work on the Apple Watch.”
The agency worked with Apple during software development and testing, added Gottlieb and Shuren.
Earlier this year MD+DI reported that Apple was working on an FDA submission.
